seqcml-proficiency-testing-sediment
SEQC-ML: first accredited for proficiency testing with urinary sediment microscopy imaging
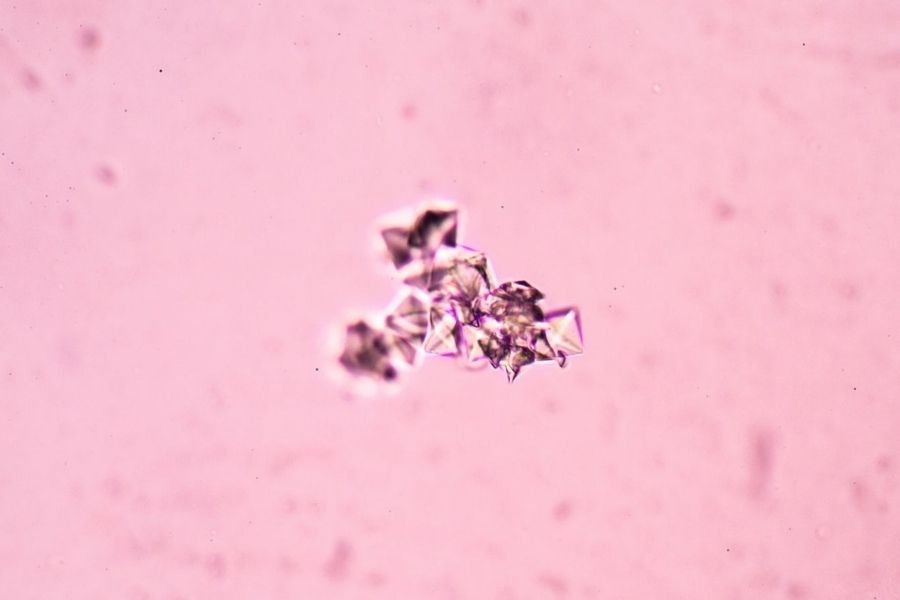
Sociedad Española de Medicina de Laboratorio (SEQC-ML) has obtained ENAC's accreditation for new proficiency testing aimed at medical laboratories on drugs, tumor markers, hormones, and urinary sediment, becoming the first accredited body for clinical cases on urinary sediment microscopy imaging. With this accreditation, SEQC-ML already has 8 accredited proficiency testing programs in biochemistry.
To obtain accreditation in the urinary sediment program, SEQC-ML has demonstrated compliance with the requirements of the reference standard for proficiency testing providers accreditation, UNE-EN ISO/IEC 17043, ensuring that the entire management process; from selecting representative images; sending clinical cases to participants; assigning consensus values; up to processing and interpreting the data, is carried out with a high level of technical competence.
Dr. Antonio Buño, president of SEQC-ML, explains in this interview what has led them to continue their firm commitment, that began years ago, to accreditation for their proficiency testing services and the benefit it brings to both laboratories and users of diagnostic services.
SEQC-ML already has several programs accredited by ENAC. What has led you to continue backing the expansion of these accreditations?
The Spanish Society of Laboratory Medicine (Sociedad Española de Medicina de Laboratorio, SEQC-ML) has been offering proficiency testing for medical laboratories for more than 40 years. It began with a first serum biochemistry program, in which 20 magnitudes were assessed and about 150 laboratories participated. The evolution of the proficiency testing programs has been constant, and currently SEQC-ML offers a total of 60 programs, covering more than 350 magnitudes. In parallel, the volume of participating laboratories has also increased, as now more than 700 laboratories work with us, making a total volume of around 8,000 registrations.
But the evolution of the programs and their growth has not only occurred in this sense, but qualitatively there has also been very important progress over the years. SEQC-ML has established offering programs of the highest quality as one of its key objectives.
Following SEQC-ML's strategic line of a constant push towards accrediting medical laboratories for the UNE-EN ISO 15189 standard and, with the aim of helping the participating laboratories in this process. At the beginning of 2020, the serum program was accredited according to the UNE-EN ISO/IEC 17043 standard, which was a very important qualitative leap. From that moment, our organization has continued to work steadily to expand the scope of accreditation, and currently has eight accredited proficiency testing programs: Serum, Proteins, Hemoglobin A1c, Blood Gases – POCT, Hormones I, Drugs, Tumor Markers and Urinary Sediment.
What challenges has SEQCML faced from implementing a quality management system in accordance with the UNE-EN ISO/IEC 17043 standard?
The accreditation project, carried out by Berta Piqueras, Montse Ventura, Sandra Bullich and Mariona Panadès, has been a very important challenge for our organization that, together with the need to implement changes, has enabled us to improve the service we offer to medical laboratories.
To comply with the requirements of the accreditation standard, for example, the statistical calculations that are made have been modified, as well as the reports that are sent to the participating laboratories to assess their performance, and the calculations and reports have been redesigned to make them more complete. In relation to the control material used in the proficiency testing programs, studies are carried out to assess its homogeneity and stability, counting on the collaboration of laboratories accredited by the UNE-EN ISO 15189, which carry out these clinical analysis with demonstrated technical competence.
Another great challenge has been to obtain accreditation for the Urinary Sediment program. We are particularly pleased to have managed to accredit this program, which works by sending clinical cases to laboratories, and which enables us to assess the performance of laboratories to identify the different form elements that are present in urine.
Progressively expanding the scope of accreditation has also been a challenge and, in turn, a great satisfaction. SEQC-ML is currently the only proficiency testing provider in Spain which has accredited programs covering the main areas of biochemistry.
What assurance do accredited proficiency testing programs provide to laboratories that use your services?
The reference standard for the proficiency testing providers’ accreditation, UNE-EN ISO/IEC 17043, guarantees that the entire management process, from preparing the control material; analyzing the samples to guarantee their homogeneity and stability; sending them to the participants, to the data's treatment and interpretation is carried out with a high level of technical competence, which ensures the participating laboratories' performance is correctly assessed. In this line, SEQC-ML’s commitment is to offer proficiency testing programs of the highest quality through our accreditation, therefore guaranteeing technical competence in all the processes involved in carrying them out.
The recruitment of accredited proficiency testing providers offers laboratories the guarantee of knowing that their technical competence is endorsed by an independent third party - an accreditation body and, therefore, they do not need to develop a methodology or invest resources to assess them with their own means. In addition, having accredited programs is important for those laboratories that are accredited or wish to start the path towards accreditation according to the UNE-EN ISO 15189 standard. From SEQC-ML, we are clearly committed to accrediting medical laboratories according to this standard, and we advocate its mandatory compliance, since it represents a crucial improvement in the laboratories’ technical competence.
What value do you consider these accredited proficiency testing services provide to diagnostic quality and the patients?
From the laboratory's individual point of view, proficiency testing programs are an essential tool to understand and improve the reliability of the results. For this reason, having accredited programs is essential for detecting possible errors or deviations from the measurement methods' usual behaviour, and simultaneously for identifying areas for laboratory improvement to obtain reliable analytical results that enable a diagnosis to be confirmed or discarded, and to carry out a correct follow-up of patients.
From the collective perspective, proficiency testing exercises play a very important role in monitoring the analytical performance of the available measurement methods, as well as how adequate these benefits are for the intended clinical use. In this sense, establishing a reliable and real vision of the laboratories' collective state of the art enables not only them, but also the in-vitro diagnosis (IVD) medical device providers, to move towards withdrawing obsolete methods and their general improvement.
For all these reasons, SEQCML considers it essential to have ENAC's accreditation, both for the proficiency testing providers, according to the UNE-EN ISO/IEC 17043 standard, and for the medical laboratories themselves, in this case according to the UNE-EN ISO 15189, to ensure the laboratories' and their suppliers' technical competence, which enables them to gain the maximum benefits possible in the accredited laboratories and provides greater patient safety.
Accreditation News
Accreditation News is published quarterly and sent to organizations and to people who have asked to be included on its mailing list.
Would you like to receive a free copy of Accreditation News? Subscribe here.

